Straws
 |
History of Drinking Straws:
Marvin Chester Stone was feeling thirsty. Winding down after a long day's work, he sipped a mint julep at his home off 9th Street in Washington, D.C. But something was getting in his way. More particularly, something was getting in his drink. It was an unwelcome reedy residue. It was his straw.
His straw was shedding.
This was the 1880s, when gentlemen sipped their whiskey through long tubes made of natural rye that lent a grassy flavor to whatever drink they plopped in. For many centuries, it was not uncommon for a sot to order a gin and tonic and wind up drinking a gin and tonic infused with natural grass flavors. Stone didn't have much patience when it came to non-mint plants floating around in his mint julep, and did something radical that billions of people around the world have appreciated in the 130 years since. He reinvented the straw.
But who drinks soda or water from wax-paper tubes these days? Approximately nobody. The man who invented the bendy straw -- the straw you know with the flexible elbow that bends like a tiny accordion -- wasn't born until about two decades years after Stone's seminal blow-up over grass getting into his mint julep. But before we move forward a few decades, let's go back a few millennia.In his first try, he wound paper around a pencil to make a thin tube, slid out the pencil from one end, and applied glue between the strips. Voila: paper straw! Also: glue? This was a halfway solution. Stone refined it by building a machine to wind paper into a tube and coat the outside with a paraffin wax to keep it from melting in bourbon. He patented the product in 1888. Today, Marvin Chester Stone is considered the godfather of the straw.
5,000 YEARS OF STRAWS
Here is a short history of the drinking straw in 30 seconds. Historians don't know what civilization first came up with the idea of sticking tubes into cups and slurpling, but the earliest evidence of straws comes from a seal found in a Sumerian tomb dated 3,000 B.C. It shows two men using what appear to be straws taking beer from a jar. In the same tomb, archeologists also found history's first known straw -- a tube made from gold and the precious blue stone lapis lazuli.It's unlikely that Sumerians created the ur-straw all by themselves. The metal straw Argentinians use to drink mate (sometimes called a bombilla) is known be centuries old, at least. In the 1800s, when the rye grass straw came into vogue, its virtues -- cheapness and softness -- were also its vices, as it had a tendency to come apart in liquid. There have been two major straw innovations in the last 150 years. First, Stone made the straw dependable.
Second, someone else made the straw bendable.
HOW THE STRAW BENT
The city of San Francisco has 3,500 restaurants today, more per-person than any metro in the U.S. If we assume that almost all of them buy, stock, and serve some of the billions of plastic bendy straws produced in the U.S. every year, San Francisco could be America's per-capita bendy straw capital. This would only be appropriate, considering that the bendy straw was born at a milkshake counter of a Bay-area restaurant.Half a century after Marvin Chester Stone found grass in his julep, Joseph B. Friedman was sitting at his brother's fountain parlor, the Varsity Sweet Shop, in the 1930s, watching his little daughter Judith fuss over a milkshake. She was drinking out of a paper straw, so we can be assured that the milkshake did not taste like grass. But since Stone's paper straw was designed to be straight, little Judith was struggling to drink it up.
Friedman inserted a screw into the straw toward the top (see image). Then he wrapped dental floss around the paper, tracing grooves made by the inserted screw. Finally, he removed the screw, leaving a accordion-like ridge in the middle of the once-straight straw. Voila! he had created a straw that could bend around its grooves to reach a child's face over the edge of a glass.
The modern bendy straw was born. The plastic would come later. The "crazy" straw -- you know, the one that lets you watch the liquid ride a small roller coaster in plastic before reaching your mouth -- would come later, too. But the the game-changing invention had been made. In 1939, Friedman founded Flex-Straw Company. By the 1940s, he was manufacturing flex-straws with his own custom-built machines. His first sale didn't go to a restaurant, but rather to a hospital, where glass tubes still ruled. Nurses realized that bendy straws could help bed-ridden patients drink while lying down. Solving the "Judith problem" had created a multi-million dollar business.
***
If straws that bend aren't quite the pinnacle of modern innovation, theirs is still a textbook story of invention. The drinking tube is practically as old as history. But only in the last century-and-a-half did two tweaks lead us to the simple stick of bendy plastic you unwrap every time you grab a seat at a diner. The smallest features of modern life are stealth inventions. Their ingenuity is quiet. Their advantages are imperceptible. But they are inventions.Friedman had to fight to prove the urgency of his invention. In his legal claim to the U.S. Patent Office in 1936, he summed up the tweaker's manifesto in a short paragraph that makes a fitting capstone to this brief history of the bendy straw:
Applicant has met a problem long existing in the art. A view of any soda fountain on a hot day, with the glasses showing innumerable limp and broken straws drooping over the edges thereof, will immediately show that this problem has long existed. Where we have the conditions where certainly the straw is old, where corrugated tubing is old, and where no inventor, during those years, has seen fit or has been able to solve this problem, whereas applicant did, that situation alone is prima facie evidence of invention. 1
Glue
It is estimated that about 40 lb (18.2 kg) per year of glue are used for every person in America, and it is easy to see how and why when one looks at the extent of uses. Furniture, plumbing, shoes, books, buildings, and automobiles all use glue in some part of their construction.
Glues are part of a larger family called adhesives. The two classes are distinguished by the fact that glue comes from organic compounds while adhesives are chemical-based. Adhering materials called epoxies, caulks, or sealants are also chemical compounds that have special additives to give them properties suitable for particular jobs or applications.
Glue came into being when ancient tribes discovered that the bones, hides, skin, sinew, and other connective tissues from animals could be processed to remove collagen, the protein in these tissues. The collagen was sticky and was useful for holding things together. Milk solids, known as casein, and blood albumin can also be used as a basis for glue. Dried serum from cows' blood yields albumin that coagulates (clumps together) when it is heated and becomes insoluble in water.
Fish glue was also made from the heads, bones, and skin of fish, but this glue tended to be too thin and less sticky. By experimenting, early man discovered that the air bladders of various fish produced a much more satisfactory glue that was white and tasteless. It eventually was named isinglass or ichthocol.
There are three classes of substance that are called glues and that do not contain chemicals, compounds, or high-tech additives; these are bone glue, hide or skin glue, and fish glue. Technically, other sticky substances are adhesives, gums, or cements, although consumers tend to use these terms interchangeably.
Plants have also been used to produce glues collectively called vegetable glues. These materials are dispersible or soluble in water and are usually made from the starches that compose many grains and vegetables. The natural gums include agar, from colloids in marine plants, algin that is derived from seaweed, and gum arabic, an extract of the acacia tree (also known as the gum tree). The substance called marine glue is used to caulk seams, but it consists of tar or pitch and is not truly a glue.
History
The earliest evidence of use of glue can still be observed in the cave paintings made by our Neanderthal ancestors in Lascaux, France. These early artists wanted their work to last and mixed glue with the paint they used to help the colors resist the moisture of the cave walls. Egyptian artifacts unearthed in their tombs show many uses of glues; perhaps the most striking are the veneers and inlays in wood furniture, which was made using glue as early as 3,000 B.C. The Egyptians also used glue to produce papyrus. Greek and Roman artists used glues extensively; mosaic floors and tiled walls and baths are still intact after thousands of years.
Furniture-making relies heavily on glues. Although there are many techniques for fastening pieces together, glue is often used either permanently or to align pieces while other connections are put in place. All of the great cabinetmakers from the sixteenth through the nineteenth centuries used glue in furniture construction, including Chippendale, Hepplewhite, Duncan Phyfe, the Adams brothers, and Sheraton. The glues used by these cabinet makers were made from animal hides, hooves, and other parts that had been reduced to jelly, then dried. The jelly was ground into power or flakes. It was remixed with water and heated gently in a glue pot. This product was brown, brittle, hard, and not waterproof. Yet this glue was the only glue available until World War I. At that time, casein glues made of milk and nitrocellulose glues were first manufactured.
In the 1930s, advances in the chemical and plastics industries led to development of a wide range of materials called adhesives and plastic or synthetic resin glues. World War II led to a further flowering of this industry when neoprenes, epoxies, and acrylonitriles were invented. These were used by the military and were not available for commercial use until the late 1940s or 1950s. Since that time, highly specialized, waterproof adhesives have been developed for many industries and unique applications including construction of the Space Shuttle. Glues are still used in woodworking and the manufacture of abrasives like sandpaper. They are also used as a colloid in industrial processes; colloids are added to liquids to cause solid particles that are suspended in the liquid to separate out so they can be recovered, either to clean the liquid or process the solids.

Peter Cooper
Best remembered as a philanthropist, Peter Cooper was a prolific inventive genius and a highly successful manufacturer. Cooper was born in New York City, the son a Revolutionary army soldier who was active in numerous enterprises and involved young Peter in all of them. Although Cooper had only one year of formal education, his early experiences with his father prepared him for success in his varied business career. Apprenticed to a coachmaker at the age of 17, Cooper did so well that his employer paid him a salary and offered to back him in his own enterprise. Instead, Cooper went into the cloth-shearing business, in which he prospered. He then bought the rights to a glue-making process, improved it with his own invention, began operating a glue factory, and secured a virtual monopoly of the American glue business.
In 1828 Cooper moved into iron manufacturing, building the Canton Iron Works in Baltimore, Maryland, intending to supply the Baltimore & Ohio Railroad. The railroad was on the verge of failure, however, because of the twisting and hilly route its tracks followed. Most engineers at that time held that locomotives couldn't run on such terrain. Cooper promptly built America's first steam locomotive, which was small but powerful. In 1830, this "Tom Thumb" pulled 40 passengers at a speed of 10 miles per hour and proved that railroads could run on track that curved.
Cooper's business enterprises grew rapidly after this success. His iron business expanded into mines, foundries, wire manufactories, and rolling mills. In 1854, Cooper's Trenton factory produced the first iron structural beams for use in erecting fireproof buildings. Cooper became a principal backer and unwavering supporter of Cyrus Field's (1819-1892) project for laying the Atlantic telegraph cable. As president of the North American Telegraph Company, Cooper owned and controlled half of the telegraph lines in the United States. As an inventor, Cooper designed an early washing machine and various engines for powering watercraft.
Raw Materials
Glue manufacturers obtain bones and tissues of animals from slaughterhouses, tanneries, and meat packing companies; it is no coincidence that the world's largest glue manufacturer is the dairy called Borden Company. The animal remains that are the raw materials for glue may include ears, tails, scraps of hide or skin, scrapings from the fleshy sides of hides, tendons, bones, and feet. Similarly, manufacturers of fish glue obtain bones, heads, scales, and skins of fish from canneries and other processing plants.
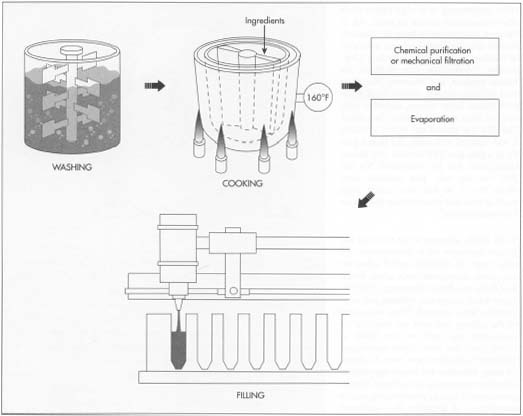
The Manufacturing
Process
Making hide or skin glue
1. With only minor variations, the same basic processes are used to make bone glue, hide or skin glue, and fish glue. The hides and other scraps are washed so that dirt is removed, and they are soaked to soften them. This material is called stock, and it is passed through a series of water baths in which more and more lime is added to make the hides and skins swell and break them down. The swollen hides are rinsed in a large washing machine to remove the lime. The last traces of lime are eliminated by treating the stock with weak acids like acetic or hydrochloric acid. Finally, the stock is cooked either by boiling, it in open tanks or cooking it under pressure in autoclaves.
2. Cooking at the correct temperature and for the right length of time breaks down the collagen and converts it into glue. If the temperature or timing is off, the quality of the glue will be ruined. Large steam coils in the open tanks heat the water and product to 160°F (70°C). Three or four treatments with clean water are performed at increasing temperatures (or pressures if a pressurized system is used). The resulting liquid, called "glue liquor" is extracted and reheated again to thicken the glue.
3. When cooled, this material looks like jelly and is solid; although it looks like the kind of gelatin used in food, it contains impurities. To remove the impurities and make the glue clear, chemicals like alum or acid followed by egg albumin may be added. These chemicals cause the impurities to precipitate, or fall out, of the glue. Mechanical methods can also be used to clean the glue. These include passing the glue through a series of mechanical filters or through paper filters or ground bone called bone char.
4. Different additives are mixed with the glue liquor to make brown, clear, or white glue. Sulfurous acid, phosphoric acid, or alum are among these additives. Zinc oxide is added to produce white "school glue."
5. To this point, the glue is a weak, runny liquid. It is made more concentrated in vacuum evaporators and dried in one of several methods. The glue can be chilled into either sheets or blocks then suspended on nets to dry and become still more concentrated. The glue can also be dropped as beads or "pearls" into a non-water bearing liquor that further dries the concentrated beads. The pearls, blocks, or sheets are then mixed to the right consistency and pumped into bottles or jars for sale.
These days the word ‘Mylar’ has been very common. Almost every person is using it. No one knows exactly what its meaning is, but almost everyone use it to signify the plastic. It is common to talk about it whenever we are talking about the plastic sheets or when we are talking about the polyester films. It is none of these.
It is unique in its chemical composition. It is not even the chemical name of the compound it represents. It is simply a trade name to signify a family of plastics, which are made from the PET or the Polyethylene Terephalate.
Hence due to its chemical composition, it is often mistaken for the polyester or sometimes the plastic sheets. Plastic film is not a narrow term. It is used for a broader meaning. It is basically used to signify any kind of polyester material, which have been cast, calendared or extruded in a slender as well as broad film. These materials are then referred to as the plastic film. There are a number of plastic films, which are in use today. Almost all of them have their own chemical composition and they have their own particular use.
They are being manufactured in a range of finishes and styles. Among them comes the Mylar sheet. There a number of Mylar sheets available in the market today. Each of them bears some kind of unique property. They all have their own feature. They come in different shapes and thickness. They are Anti static, plain, adhesion treated and coated with barrier. There is a range of finishes as well. They include finishes like white, low, clear and moderate. They also include black and haze. Like its finishes, there is different thickness available in the Mylar sheets. Its thickness varies from the 0.0005” to the 0.014”.
This thickness is very low as compared to what we see today. But this thickness is suitable for the kind of jobs being done by the Mylar sheets. There are a number of their uses. These include works like printing, casting; die cutting, barrier shield, defensive overlay clothing and a number of other applications as well. Besides, it is also used in a number of other places. These are used in coil winding, insulations and sometimes in cryogenic window. For all of its uses , its physical property is one of the biggest driving factors. These are a mark for growth. 3
, its physical property is one of the biggest driving factors. These are a mark for growth. 3
Styrofoam™ cups is what cups made out of polystyrene foam, or plastic #6, are commonly referred to. However, the term Styrofoam™ is a trademark of the Dow Chemical Company and is not used to make food products -- including cups, plates, take-out containers or egg cartons.Styrofoam™ is used for packaging materials such as foam peanuts or inserts in boxes around electronic equipment, along with materials for crafts.
Despite the fact that the term Styrofoam™ cups is technically incorrect, most people refer to these polystyrene cups that way. Most people purchase disposable cups because they are easy to clean up and do not need to be washed. If you stop and get a cup of coffee on the way to work in the morning, chances are it came in a polystyrene cup.
There is much debate over what type of disposable cups and plates are better for the environment. In general, if you can use a cup or plate made of glass or ceramic that can be washed and reused, that is the best choice. If disposable plates and cups are a necessity, though, most environmentalists advocate choosing paper first, then plastic. Styrofoam™ cups should generally be thought of as a last resort. 4
Polystyrene is a strong plastic created from erethylene and benzine that can be injected, extruded, or blow molded; making it a very useful and versatile manufacturing material. However, do not forget to recycle polystyrene products.
Most of us recognize polystyrene in the form of styrofoam used for beverage cups and packaging peanuts. However, polystyrene is also used as a building material, with electrical appliances (light switches and plates), and in other household items.
Eduard Simon & Hermann Staudinger Polymer ResearchPolystyrene has a long history of evolution behind it. In 1839, a German apothecary called Eduard Simon discovered polystyrene. Eduard Simon isolated the substance from natural resin, however, he did not know what he had discovered.
It took another German, organic chemist, Hermann Staudinger, to realize that Simon's discovery, comprised of long chains of styrene molecules, was a plastic polymer.
Making bone glue
Manufacture of bone glue is somewhat more complicated. Bones are processed most often in pressure tanks, but additional processing is needed to remove the minerals. The bones are degreased with solvents, then hydrochloric acid in an 8% solution is applied to the bones. The acid removes calcium phosphate and other minerals and leaves collagen in the same shape as the piece of bone. The acid is removed from the collagen, and it is dried to produce commercial-grade ossein or bone protein (also termed acidulated bone) that is the basis for bone glues. After the ossein is created, it can then be processed in the open-tank method and the subsequent steps used to make glue from hides, as described above.
Quality Control
All processes in the manufacture of glue are monitored carefully using instruments, computerized controls, and observation. Improper temperatures or pressures will ruin large quantities of stock that must then be wasted; manufacturers will not risk such errors.
Safety and sanitation are also major concerns. Glue manufacturers tend to be located very close to supplies of hides and other raw materials to prevent disease, vermin, contamination, and major costs like transportation. Workers' safety is carefully monitored, as is the production of a pure glue.
Byproducts/Waste
Glue itself is a byproduct of dairies, meat processing plants, and other facilities that generate the raw materials needed for glue production.
The Future
Glues are essential to our future. More and more manufacturing processes are using various forms of glue (and including adhesives) to replace stitching, stapling, and more expensive (and less effective) forms of fastening. Experiments with medical glues suggest that one-third of all wounds may be "stitched" with glues in the next few years. Glues have proven to be so versatile that scientists are constantly watching for new applications that will make our lives simpler. 2
Mylar
These days the word ‘Mylar’ has been very common. Almost every person is using it. No one knows exactly what its meaning is, but almost everyone use it to signify the plastic. It is common to talk about it whenever we are talking about the plastic sheets or when we are talking about the polyester films. It is none of these.
It is unique in its chemical composition. It is not even the chemical name of the compound it represents. It is simply a trade name to signify a family of plastics, which are made from the PET or the Polyethylene Terephalate.
Hence due to its chemical composition, it is often mistaken for the polyester or sometimes the plastic sheets. Plastic film is not a narrow term. It is used for a broader meaning. It is basically used to signify any kind of polyester material, which have been cast, calendared or extruded in a slender as well as broad film. These materials are then referred to as the plastic film. There are a number of plastic films, which are in use today. Almost all of them have their own chemical composition and they have their own particular use.
They are being manufactured in a range of finishes and styles. Among them comes the Mylar sheet. There a number of Mylar sheets available in the market today. Each of them bears some kind of unique property. They all have their own feature. They come in different shapes and thickness. They are Anti static, plain, adhesion treated and coated with barrier. There is a range of finishes as well. They include finishes like white, low, clear and moderate. They also include black and haze. Like its finishes, there is different thickness available in the Mylar sheets. Its thickness varies from the 0.0005” to the 0.014”.
This thickness is very low as compared to what we see today. But this thickness is suitable for the kind of jobs being done by the Mylar sheets. There are a number of their uses. These include works like printing, casting; die cutting, barrier shield, defensive overlay clothing and a number of other applications as well. Besides, it is also used in a number of other places. These are used in coil winding, insulations and sometimes in cryogenic window. For all of its uses
 , its physical property is one of the biggest driving factors. These are a mark for growth. 3
, its physical property is one of the biggest driving factors. These are a mark for growth. 3Styrofoam Cups
Styrofoam™ cups is what cups made out of polystyrene foam, or plastic #6, are commonly referred to. However, the term Styrofoam™ is a trademark of the Dow Chemical Company and is not used to make food products -- including cups, plates, take-out containers or egg cartons.Styrofoam™ is used for packaging materials such as foam peanuts or inserts in boxes around electronic equipment, along with materials for crafts.
Despite the fact that the term Styrofoam™ cups is technically incorrect, most people refer to these polystyrene cups that way. Most people purchase disposable cups because they are easy to clean up and do not need to be washed. If you stop and get a cup of coffee on the way to work in the morning, chances are it came in a polystyrene cup.
There is much debate over what type of disposable cups and plates are better for the environment. In general, if you can use a cup or plate made of glass or ceramic that can be washed and reused, that is the best choice. If disposable plates and cups are a necessity, though, most environmentalists advocate choosing paper first, then plastic. Styrofoam™ cups should generally be thought of as a last resort. 4
Polystyrene is a strong plastic created from erethylene and benzine that can be injected, extruded, or blow molded; making it a very useful and versatile manufacturing material. However, do not forget to recycle polystyrene products.
Most of us recognize polystyrene in the form of styrofoam used for beverage cups and packaging peanuts. However, polystyrene is also used as a building material, with electrical appliances (light switches and plates), and in other household items.
Eduard Simon & Hermann Staudinger Polymer ResearchPolystyrene has a long history of evolution behind it. In 1839, a German apothecary called Eduard Simon discovered polystyrene. Eduard Simon isolated the substance from natural resin, however, he did not know what he had discovered.
It took another German, organic chemist, Hermann Staudinger, to realize that Simon's discovery, comprised of long chains of styrene molecules, was a plastic polymer.
In 1922, Hermann Staudinger published his theories on polymers, stating that natural rubbers were made up of long repetitive chains of monomers that gave rubber its elasticity. He went on to write that the materials manufactured by the thermal processing of styrene were similar to rubber. They were the high polymers including polystyrene. In 1953, Hermann Staudinger won the Nobel Prize for Chemistry for his research. 5
The marketing genius who brought us the toothpick.
Charles Forster was a marketing genius who might have sold a side of beef to a vegetarian. He was born in 1826 in Charlestown, Mass., into an old and aristocratic New England family. While working for his uncle's import/export business in Brazil, he noticed that the natives had beautiful teeth, which he attributed to their use of handcrafted toothpicks. At a time when virtually everything was becoming mass produced, Forster vowed to make a fortune producing wooden toothpicks so cheaply by machine that he could export them to South America.
Forster himself was not mechanically inclined, but he had the business savvy to acquire the rights to a patent that gave him a monopoly on a toothpick-making process. It was a byproduct of the work of Boston inventor Benjamin Franklin Sturtevant, whose own passion was making shoes by machine. At the time, most shoes were put together with wooden pegs, and the weak link in the operation was supplying pegs of uniform quality. This led Sturtevant to concentrate on producing long strips of knife-edged veneer from which pegs could be sliced off. Forster saw that toothpicks could be made in much the same way, and by 1870, his operation was capable of producing millions of toothpicks per day. But he could not find a market for them in Boston.
The Yankee tradition was to whittle a toothpick on demand. It did not make sense to spend money on something one could make for oneself, let alone for something that would be used once and then discarded. But Forster came up with ingenious marketing schemes.
He first targeted stationers, who dealt in small items. When he could not place his product in their stores, he hired personable young people to go to those same retailers and ask for wooden toothpicks. Naturally, the retailers had to turn away the potential customers. Shortly afterward, Forster would make return visits to the stores, where he easily sold his wares. To reinforce the wisdom of the shopkeeper's decision, Forster's shills soon came back to ask again for toothpicks, and this time the sales were made. The boxes of toothpicks were then returned to Forster, who could resell them to the retailer, who now was prepared to talk them up to real customers.
To get toothpicks into restaurants, Forster hired Harvard men. After they had finished dining on Forster's dime at a local establishment, such as the Union Oyster House, they demanded wooden toothpicks. When they were told none were available, the students raised a ruckus and vowed never to eat there again. Naturally, when Forster came around some days hence, the restaurant manager purchased boxes of toothpicks to distribute to his customers.

Once wooden toothpicks became readily available in restaurants, diners picked them up on their way out and used them for their intended purpose. After they were used to clean the teeth, the toothpicks had a further use. Chewing toothpicks in public soon became fashionable among well-to-do men, and after a while young women began taking up the practice. One Bostonian observed that at lunchtime "nearly every third woman met in the vicinity of Winter and West streets has a toothpick between her lips." This ostentatious primary and secondary toothpick usage in the 1870s served to further the general desire for toothpicks.
It was a common observation of the time that many of the young men standing in front of a good hotel chewing toothpicks were suggesting they had eaten in its fine dining room, when in fact they could not afford to do so. In time, chewing a toothpick anywhere became a sign of contentment and insouciance. In his Life on the Mississippi, Mark Twain described feeling that he knew the river so well that he found himself cocking his cap and "wearing a toothpick" while at the wheel of his riverboat.
Thus, the toothpick took on a life of its own, serving not only as a utilitarian object but also as a status symbol and even as an accessory. While Charles Forster may never have dreamed that his toothpicks would have such unintended ancillary uses, he would no doubt have welcomed them as extensions of his initial marketing efforts.
Glass Sheets
History of Glass
The mysterious physical, optical and aesthetic properties of glass have always intrigued man. Even the most sophisticated 20th century man is amazed and bemused by this solid, which he has been told is really a rigid uncrystallized liquid. The product and the process used to manufacture it seem to smack of alchemy, for glass is nothing but coarse sand and soda ash transformed into smooth transparent forms.
According to the Roman historian Pliny, who wrote in Naturalis Historica in 77 A.D., man first produced glass by accident about the year 5000 B.C. Phoenician sailors feasting on a beach near Belus in Asia Minor, could find no stones on which to place their cooking pots; therefore, they set them on blocks of soda carried by their ship as cargo. As the fire's heat increased, the sand and soda turned to molten glass.
Pliny's anecdote now is considered apocryphal, but it contains an accurate recipe for producing glass: heat plus silica and soda ash.
Ornamental glass beads dating from 2500 B.C. have been found in Egypt, and glass rods from even earlier have been uncovered in Babylon. The first useful glass objects date to Egypt's 18th dynasty, about 1500 B.C. Egyptians attached metal rods to silica paste cores, which they dipped repeatedly into molten glass to produce small bottles. The cores later were removed. The goblet of Thutmose III, made about 1490 B.C. and now at New York's Metropolitan Museum of Art, was produced in this manner.
Glassblowing, a Babylonian discovery, probably came about when glassmakers using the core-dipped method switched to hollow metal rods to hold silica paste cores and then discovered that molten glass could be blown into shapes. After this discovery, which dates to about 250 B.C., glass vessels suddenly became easy and inexpensive to produce. Romans imported Syrian and Babylonian glassmakers, and small bowls and bottles were selling for only a Roman penny in 200 B.C. Pliny the Elder noted in 79 B.C. that fine glass cups were replacing cups of precious metals as a status symbol among the Roman rich.
Glass, however, did not replace shutters at the windows of Roman homes. The Romans tried but failed to cast transparent flat glass to enclose or ornament their homes. Slabs 1/2" thick have been excavated - including a 32 by 44-inch piece at Pompeii - but Romans did not discover the art of grinding and polishing cast glass to make it transparent. Instead of glass, the rich used thin, translucent sheets of alabaster to enclose wall openings.
With the breakdown of the Roman Empire, glassmaking technology stagnated in Europe; in fact, it almost disappeared. True, Gothic cathedrals of the late 12th century and later featured brilliant bits of colored glasses, complex designs and rate and were prohibitively expensive. Even the rich still shuttered their windows, and the Middle English word for windows - "wind eyes" - underlined the fact that wall openings enclosed in glass were, for all practical purposes, nonexistent.
During the 13th and 14th centuries, glassmaking was revived in Venice as a result of that Italian state's trade contacts with Byzantium. Soda-Lime was developed by glassmakers of the island or Murano in about 1450, and Venetians termed this clear, thin glass cristallo. Despite attempts to keep their technology secret, it soon spread north over the Alps to Germany, France, Belgium and England.
In England, where deforestation was a problem as early as the 15th century, glassmakers were required after 1615 to use coal instead of wood in the glassmaking process. About 1675, the English learned to add lead oxide to the basic glass formula, and the resulting solid, heavy and durable vessels progressively replaced the fragile glasses of Venice.
Flat glass for windows was still rare during much of the 17th and 18th centuries. Small panes were made by blowing a large glob of glass, removing it from the blowing iron and then rotating the glass quickly so it would spread and flatten. Such glass had a dimple in its center, many air bubbles and a pattern of concentric circles, but it was transparent and effective in keeping out the weather. At the end of the 17th century, the French learned how to grind and polish cast glass to produce plate glass, but only the rich could afford it.
During the 1800s, glass technology improved rapidly. A hand-operated split mold developed in 1821 that ended the age of blowing individual bottles, glasses and flasks. A semi-automatic bottle machine perfected 50 years later mass-produced bottles and turned them into the everyday miracle they are today.
Great strides were made in the manufacture of flat glass during the 19th century. Compressed air technology led to flatter, better glass panes. Controlled amounts of air were used to blow a large glass cylinder, which was slit lengthwise, reheated and allowed to flatten under its own weight. Large, relatively inexpensive lites of glass were produced in this manner. As a result of such technological advances, window areas that required 18 to 24 panes to enclose in 1730 could be increased dramatically and glass prices dropped by the 1860s, glass-enclosed "wind eyes" were commonplace in the humblest homes.
Plate glass, that wickedly expensive French product, also became commonplace by the end of the 19th century. Water power, then steam and then electricity made the grinding and polishing of heavy glass plates faster and easier. By the 1860s, smart stores and office buildings in Europe and North America glistened with plate glass. France, Belgium and Germany monopolized the production of the product until 1883, when the Pittsburgh Plate Glass Company became the first successful manufacturer of the product in the United States. By 1895, the company could produce 20 million square feet of plate glass a year, and imports from Europe fell sharply.
With the 20th century came an era of revolutionary technology. Machines were developed, improved and perfected to produce endless ribbons of sheet (window) glass, to produce plate glass polished and ground simultaneously on both sides and to produce float glass on a bed of molten tin. Also developed were processes to strengthen glass through thermal and chemical tempering, to add tints to glass for reduced heat transmission and glare and to coat glass with transparent metal and metal oxide films that reflected heat or conducted electricity. And products marrying these processes and developments were created to help make life more convenient, more comfortable, safer and more beautiful.
In retrospect, the romance of glass is not an Egyptian producing a bottle for a Pharoah or window glass being made from a cylinder, a pane at a time, in a one-man glass house. The true romance of glass is the story of the reasonable cost for use in architecture, transportation, industry, science and the home. Billions of people now benefit because technology has made glass a versatile, easy-to-use miracle.
The Nature of Glass
Glass is not easily described. Its physical structure does not conform to liquid, solid or gas. Glass actually is more of a liquid than the solid it appears to be. Its complex nature has intrigued man from ancient times.
The American Society for Testing and Materials defines glass as "an inorganic product of fusion which has cooled to a rigid condition without crystallizing". Glass can be considered, then, an unusual material which has the random atomic arrangement of liquid but which somehow has been "frozen" in place so that it is a solid and permanent substance. Glass can be transparent, translucent or opaque. It is non-porous, non-absorptive and impervious to the common elements and many harsh chemicals and liquids. It is exceptionally resistant to abrasion and surface scratches. It is one of the best electrical insulation materials, yet can be treated to conduct electricity. Glass has lower head conductivity than most metals and can possess a very low, zero or even negative coefficient of expansion. Because it contains a large proportion of silica and is produced by the action of heat upon that silica, it is generally categorized as a ceramic. Glass, however, stands in a class by itself, quite distinct from other ceramics.
Most ceramic materials are shaped cold and then fired to produce the desired result; glass is shaped at extremely high temperatures and then allowed to cool. It again may be made semi-plastic, plastic or even molten by the further application of heat. For this reason, glass also is considered a thermoplastic material, which softens when heated and hardens when cooled.
The Flat Glass Recipe
Glass goes back millenniums formed by nature as obsidian, or black glass, a hard noncorrosive, semiopaque substance fused by volcanic eruptions and enduring centuries of erosion.
This natural glass is composed of three elements of the earth-sand, soda and lime. These same elements in varying forms also make up the basic composition of manufactured glass products ranging from containers and glassware to windshields and windows for high-rise commercial buildings. About 50 other chemical elements are used in modern glassmaking, in major and minor ways, to affect color, viscosity or durability, or to impart some desired physical property. But nature's original ingredients are still basic elements in the formulation of glass.
Glass largely is an open chain of silicon atoms with atoms of various oxides occupying the spaces between. It is this loose structure that permits transparency. Silica, or sand, is the most important ingredient in glassmaking since it is the source of, and provides the structure for, transparency. But sand requires soda and lime for practical glassmaking.
Today, an average batch mix used to manufacture flat glass products contains about 70 percent silica sand, 13 percent lime, 12 percent soda and small amounts of other materials. About one-quarter of the batch is in the form of cullet, or cleaned and crushed glass recovered from previous glassmaking operations.
Silica or silicon dioxide, which is converted into glass by the action of heat is very difficult to fuse, requiring extremely high temperatures. Ancient scientists discovered that other materials such as soda, when melted in close contact with sand, would permit the melting of silica at much lower temperatures. Such materials are known as fluxes, and soda was probably the first flux.
The primary forms of soda used in glassmaking are soda ash (sodium carbonate) or caustic soda (sodium hydroxide). When a mixture of sand and soda dissolves in the molten soda, forming sodium silicate. Depending on the proportions of sand and soda, this sodium silicate is more or less soluble in water and is known as water glass. To overcome water solubility of glass, another element, lime, is required.
Lime (calcium oxide) usually is introduced into the glass batch mix in the form of limestone. Its use in correct proportion causes formulation of a soda-lime-silicate composition that is virtually unaffected by moisture or acids. Lime also renders the glass more viscous at the working temperature, shortens the setting time and improves weathering properties.
Because of its low melting range, the soda-lime-silicate composition undoubtedly was the type used by ancients to produce the earliest known vessels, vases, semi-precious glass stones and beads, and, much later, the earliest form of window glass. Today, soda-lime-silicate is the basis for float glass, and of course, products fabricated from it.
Other materials are added to produce different properties in the basic flat glass product or to replace one of the basic elements to produce different types of commercial glasses. Lead, for example, in the form of lead oxide, may be used to replace lime, and is introduced to increase brilliance, density and index of refraction. Lead glasses included optical and ophthalmic glasses and the finest stemware and art objects. Boron, substituted in whole or in part for the silica, increases the refractive index, deepens the color produced by various other coloring materials, and greatly reduces the coefficient of thermal expansion. Borosilicate glasses are used for such high heat resistant products as ovenware, laboratory glasses and range surfaces. Metallic oxides are added to produce tinted or colored glasses. 6
Rubber Bands
The History of Elastic and Rubber Bands
Ancient rubber
The ancient Mayan People used latex to make rubber balls, hollow human figures, and as bindings used to secure axe heads to there handles and other functions. Latex is the sap of various plants, most notably the rubber tree. When it is exposed to the air it hardens into a springy mass. The Mayans learned to mix the rubber sap with the juice from morning glory vines so that it became more durable and elastic, and didn't get quite as brittle. Both the rubber tree and the morning glory were important plants to the Mayan people - the latter being a hallucinogen as well as a healing herb. They two plants tended to grow close together. Combining their juices, a black substance about the texture of a gum-type pencil eraser was formed. Native peoples in the region still make rubber in the same way.
Vulcanized rubber
In 1736 several rolled sheets of rubber were sent to France where it fascinated those who saw it. In 1791, an Englishman namedSamuel Peal discovered a means of waterproofing cloth by mixing rubber with turpentine. English inventor and scientist, Joseph Priestly, got his hands on some rubber and realized it could be used to erase pencil marks on sheets of paper.
Thomas Hancock was an English inventor who founded the British rubber industry. He invented the masticator, a machine that shredded rubber scraps, allowing rubber to be recycled after being formed into blocks or rolled into sheets. In 1820, Hancock patented elastic fastenings for gloves, suspenders, shoes and stockings. In the process of creating the first elastic fabrics, Hancock found himself wasting considerable rubber. He invented the masticator to help conserve rubber. The first masticator was a wooden machine that used a hollow cylinder studded with teeth - inside the cylinder was a studded core that was hand cranked. In 1821, Hancock joined forces with the Scottish chemist and inventor of waterproof fabrics, Charles Macintosh. Together they produced Macintosh coats, or Mackintoshes, named after Charles Macintosh.
In 1823, Charles Macintosh patented a method for making waterproof garments by using rubber dissolved in coal-tar naphtha for cementing two pieces of cloth together. While he was trying to find uses for the waste products of gasworks, Macintosh discovered that coal-tar naphtha dissolved India rubber. He took wool cloth and painted one side with the dissolved rubber preparation and placed another layer of wool cloth on top.
In 1837, Hancock finally patented the masticator, when Macintosh's waterproofing patent was being challenged. In the pre-Goodyear and pre-vulcanization age of rubber age, the masticated rubber that Hancock invented was used for pneumatic cushions, mattresses, pillows and bellows, hose, tubing, solid tires, shoes, packing and springs. It was used everywhere. Hancock became the largest manufacturer of rubber goods in the world. The wooden masticator turned into a steam-driven metal machine, used to supple the Macintosh factory with masticated rubber.
This created the first practical waterproof fabric, but the fabric was not perfect. It was easy to puncture when it was seamed, the natural oil in wool caused the rubber cement to deteriorate. In cold weather the fabric became stiffer and in hot weather the fabric became sticky. When vulcanized rubber was invented in 1839, Macintosh's fabrics improved since the new rubber could withstand temperature changes.
Charles Goodyear, an American whose name graces the tires under millions of automobiles, is credited with the modern form of rubber. Before 1839, rubber was subject to the conditions of the weather. If the weather was hot and sticky, so was the rubber. In cold weather it became brittle and hard. Goodyear's recipe, a process known as vulcanization, was discovered when a mixture of rubber, lead and sulfur were accidentally dropped onto a hot stove. The result was a substance that wasn't affected by weather, and which would snap back to its original form if stretched. The process was refined and the uses for rubber materials increased as well. This new rubber was resistant to water and chemical interactions and did not conduct electricity, so it was suited for a variety of products. The process of making the rubber product improved as time went by, and now various chemicals are added before the mix is poured into molds, heated and cured under pressure.
But who invented the rubber bands?
On March 17, 1845, Stephen Perry of the rubber manufacturing company Messers Perry and Co, Rubber Co Manuf London patented the fist rubber bands made of vulcanized rubber. Perry invented the rubber band to hold papers or envelopes together.
At the present time Antoon Versteegde uses the same kind of rubber bands to fasten the bamboo poles in his transient constructions.
Rubber tapping
Latex (a natural, stretchy substance from which rubber is made) is extracted from rubber trees. Rubber trees are large trees (belonging to the spurge family, family Euphorbiaceae) that live in tropical (warm) areas. These trees are tapped for their latex, which is produced in their bark layers (latex is not the sap). The Pará rubber tree (Hevea brasiliensis) is native to South American rain forests, and grows to be over 30 m tall.
In 1876, an Englishman named Sir Henry Wickham collected about seventy thousand rubber tree seeds from the Para rubber tree (taken from the lower Amazon area of Brazil) and brought them to London, England. Seedlings were grown in London, and later sent to the East Indies, Ceylon and Singapore, where he started rubber plantations. The technique of tapping rubber trees for their latex was developed in Southeast Asia (before that, the trees were cut down to extract the rubber). Commercial rubber production now takes place in Malaysia, Thailand, Indonesia, and Sri Lanka (but not significantly in South America).
In 1877 an American named Chapman Mitchell learned to recycle used rubber into new products.
Modern rubber
Today about three quarters of the rubber in production is a synthetic product made from crude oil. World War II cut the United States off from rubber supplies worldwide, and they stepped up production of synthetic rubber for use in the war effort. There are about 20 grades of synthetic rubber and the intended end use determines selection. In general, to make synthetic rubber, byproducts of petroleum refining called butadiene and styrene are combined in a reactor containing soapsuds. A milky looking liquid latex results. The latex is coagulated from the liquid and results in rubber "crumbs" that are purchased by manufacturers and melted into numerous products.
There is only one kind of natural rubber. Because the rubber plant only thrives in hot, damp regions near the equator, so 90% of true rubber production today occurs in the Southeast Asian countries of Malaysia and Thailand and in Indonesia. Indonesia's production has dropped in recent years and new plantations were started in Africa to take up the slack. 7Tar Paper
Manufacture Of Roll Tar Paper
Roofing paper was first used in Scandinavia early as the last century, the invention being accredited to Faxa, an official of the Swedish Admiralty. The first tar and gravel roofs in Sweden were very defective. The impregnation of the paper with a water-proofing liquid had not been thought of, and the roofs were constructed by laying over the rafters a boarding, upon which the unsaturated paper, the sides of which lapped over the other, was fastened with short tacks. The surface of the paper was next coated with heated pine tar to make it waterproof. The thin layer of tar was soon destroyed by the weather, so that the paper, swelled by the absorption of rain water, lost its cohesiveness and was soon destroyed by the elements. This imperfectmethodof roof covering found no great favor and was but seldom employed.
InGermanythe architect Gilly was first to become interested in tar paper roofing, and recommended it in his architecture for the country. Nevertheless the new style of roof covering was but little employed, and was finally abandoned during the first year of the 19th century. It was revived again in 1840, when people began to take a renewed interest in tar paper roofs, the method of manufacturing an impermeable paper being already so far perfected that the squares of paper were dipped in tar until thoroughly saturated. The roof constructed of these waterproof paper sheets proved itself to be a durable covering, being unimpenetrable to atmospheric precipitations, and soon several factories commenced manufacturing the paper. The product was improved continually and its method of manufacture perfected. The good qualities of tar paper roofs being recognized by the public, they were gradually adopted. The costly pine tar was soon replaced by the cheaper coal tar. Square sheets of paper were made at first; they were dipped sufficiently long in ordinary heated coal tar, until perfectly saturated. The excess of tar was then permitted to drip off, and the sheets were dried in the air.
Theimprovementof passing them through rollers to get rid of the surplus tar was reserved for a future time, when an enterprising manufacturer commenced to make endless tar paper in place of sheets. Specialapparatuswere constructed to impregnate these rolls with tar; they were imperfect at first, but gradually improved to a high degree. Much progress was also made in theconstructionof the roofs, and several methods of covering were devised. The defects caused by the old method of nailing the tar paper direct upon the roof boarding were corrected; the consequence of this method was that the paper was apt to tear, caused by the unequal expansion of the roofing boards and paper, and this soon led to the idea of making the latterindependentof the former by nailing the sides of the paper upon strips running parallel with the gable. The use of endless tar paper proved to be an essential advantage, because the number of seams as well as places where it had to be nailed to the roof boarding was largely decreased. The manufacture of tar paper has remained at about the same stage and no essential improvements have been made up to the present.
As partial improvement may be mentioned the preparation of tar, especially since the introduction of the tar distillery, and the manufacture of special roof lacquers, which have been used for coating in place of the coal tar. As an essential progress in the tar paper roofing may be mentioned the invention of the double tar paper roof, and the wood cement roof, which is regarded as an offshoot.
The tar paper industry has, within the last forty years, assumed great dimensions, and the preferences for this roofing are gaining ground daily. In view of the small weight of the covering material, the wood construction of the roof can be much lighter, and the building is therefore less strained by the weight of the roof than one with the other kind, so that the outer walls need not be as heavy. Considering the price, the paper roof is not only cheaper than other fireproof roofs, but its light weight makes it possible for the whole building to be constructed lighter and cheaper. The durability of the tar paper roof is satisfactory, if carefully made of good material; the double tar paper roof, the gravel double roof, and the wood cement roof are distinguished by their great durability.
These roofs may be used for all kinds of buildings, and not only are factories, storehouses, and country buildings covered with it, but also many dwellings. The most stylish residences and villas are at present being inclosed with the more durable kinds; the double roof, the gravel double roof, and the wood cement roof. For factory buildings, which are constantly shaken by the vibrations of the machinery, the tar paper roof is preferable to any other.
In order to ascertain to what degree tar paper roofs would resist fire, experiments were instituted at the instigation of some of the larger manufacturers of roofing paper, in the presence of experts, architects, and others, embracing the most severe tests, and it was fully proved that the tar paper roof is as fireproof as any other. These experiments were made in two different ways; first, the readiness of ignition of the tar paper roof by a spark or flame from the outside was considered, and, second, it was tested in how far it would resist a fire in the interior of the building. In the former case, it was ascertained that a bright, intense fire could be kept burning upon the roof for some time, without igniting the woodwork of the roof, but heat from above caused some of the more volatile constituents of the tar to be expelled, whereby small flames appeared upon the surface within the limits of the fire; the roofing paper was not completely destroyed. There always remained a cohesive substance, although it was charred and friable, which by reason of its bad conductivity of heat protected the roof boarding to such an extent that it was "browned" only by the developed tar vapors. 8
Toothpicks
The marketing genius who brought us the toothpick.
Charles Forster was a marketing genius who might have sold a side of beef to a vegetarian. He was born in 1826 in Charlestown, Mass., into an old and aristocratic New England family. While working for his uncle's import/export business in Brazil, he noticed that the natives had beautiful teeth, which he attributed to their use of handcrafted toothpicks. At a time when virtually everything was becoming mass produced, Forster vowed to make a fortune producing wooden toothpicks so cheaply by machine that he could export them to South America.
Forster himself was not mechanically inclined, but he had the business savvy to acquire the rights to a patent that gave him a monopoly on a toothpick-making process. It was a byproduct of the work of Boston inventor Benjamin Franklin Sturtevant, whose own passion was making shoes by machine. At the time, most shoes were put together with wooden pegs, and the weak link in the operation was supplying pegs of uniform quality. This led Sturtevant to concentrate on producing long strips of knife-edged veneer from which pegs could be sliced off. Forster saw that toothpicks could be made in much the same way, and by 1870, his operation was capable of producing millions of toothpicks per day. But he could not find a market for them in Boston.
The Yankee tradition was to whittle a toothpick on demand. It did not make sense to spend money on something one could make for oneself, let alone for something that would be used once and then discarded. But Forster came up with ingenious marketing schemes.
He first targeted stationers, who dealt in small items. When he could not place his product in their stores, he hired personable young people to go to those same retailers and ask for wooden toothpicks. Naturally, the retailers had to turn away the potential customers. Shortly afterward, Forster would make return visits to the stores, where he easily sold his wares. To reinforce the wisdom of the shopkeeper's decision, Forster's shills soon came back to ask again for toothpicks, and this time the sales were made. The boxes of toothpicks were then returned to Forster, who could resell them to the retailer, who now was prepared to talk them up to real customers.
To get toothpicks into restaurants, Forster hired Harvard men. After they had finished dining on Forster's dime at a local establishment, such as the Union Oyster House, they demanded wooden toothpicks. When they were told none were available, the students raised a ruckus and vowed never to eat there again. Naturally, when Forster came around some days hence, the restaurant manager purchased boxes of toothpicks to distribute to his customers.

Once wooden toothpicks became readily available in restaurants, diners picked them up on their way out and used them for their intended purpose. After they were used to clean the teeth, the toothpicks had a further use. Chewing toothpicks in public soon became fashionable among well-to-do men, and after a while young women began taking up the practice. One Bostonian observed that at lunchtime "nearly every third woman met in the vicinity of Winter and West streets has a toothpick between her lips." This ostentatious primary and secondary toothpick usage in the 1870s served to further the general desire for toothpicks.
It was a common observation of the time that many of the young men standing in front of a good hotel chewing toothpicks were suggesting they had eaten in its fine dining room, when in fact they could not afford to do so. In time, chewing a toothpick anywhere became a sign of contentment and insouciance. In his Life on the Mississippi, Mark Twain described feeling that he knew the river so well that he found himself cocking his cap and "wearing a toothpick" while at the wheel of his riverboat.
Thus, the toothpick took on a life of its own, serving not only as a utilitarian object but also as a status symbol and even as an accessory. While Charles Forster may never have dreamed that his toothpicks would have such unintended ancillary uses, he would no doubt have welcomed them as extensions of his initial marketing efforts.
The phenomenon known as "usage drift" actually began a long time before Forster was making toothpicks. In 16th-century Portugal, there was an order of nuns that supported itself by making and selling confections that were sticky to the fingers and tacky to the teeth. Perhaps to maintain, and even increase, demand for their sweets, the nuns began to make wooden toothpicks, which served not only to clean the teeth after eating but also to pick up the morsels without touching them.
When used in this way with sweets or hors d'oeuvres, the toothpick is once again more than an item with which to pick the teeth. But on a cocktail platter, an ordinary wooden toothpick can appear too common. Thus, there has developed a plethora of "fancy" toothpicks. Gold and silver picks, with equally elegant holders, can stand up to any plate of delicacies. In Portugal, where making toothpicks by hand out of orangewood continues to be a cottage industry, larger and fancier ones called palitos especiales,complete with carved involutes and curly shafts, were considered more appropriate for special occasions. The wooden toothpick topped with colored cellophane—so often seen holding a club sandwich together—is a poor cousin to the Portuguese special.
Cocktails provided another opportunity for secondary toothpick use and further marketing. Indeed, the olive speared by a toothpick has acquired an iconic association with the martini. The toothpick enables the olive to be retrieved without getting the fingers wet, but some fastidious drinkers do not know what to do with the bare toothpick. One fellow, who did not want to put the wet pick down on the furniture, put it back into his drink—and then absentmindedly took it in with a sip. When he tried to cough it up, the thing got stuck in his nose, from where it was finally removed by an emergency-room doctor. A swallowed toothpick can be deadly if it punctures the intestines, certainly an undesirable usage drift. It was an ingested toothpick that killed the writer Sherwood Anderson, and some believe that peritonitis was also the cause of the untimely death of President Warren G. Harding, an inveterate toothpick user.
There are many other examples of usage drift, including the sticking of a toothpick into brownies baking in the oven to test if they are done. This popular secondary use is part of so many baking recipes that in supermarkets now, toothpicks often can be found shelved not among the toothbrushes and dental floss but next to the cake and brownie mixes.
Though readily promoted by manufacturers, usage drift is more often created by consumers of a product. People are natural inventors, and they are constantly finding new uses for common objects of all kinds. The best ideas propagate quickly through the culture and then become embraced by manufacturers as their own. Before there were Q-tips, young mothers wrapped a bit of cotton around the point of a toothpick and used it to clean out baby's ears and nose. This practice came to be recommended by ladies' magazines and advice columnists, and led to the invention of the Q-tip itself.
At first, the hard wooden stick terminating in soft cotton swabs suggested the toothpick connection, but today's Q-tip disguises its origins with a white paper body that blends almost seamlessly into the swab ends. The latest supply of Q-tips bought for our bathroom goes even further in removing the product from its ancestry and infancy. Except perhaps for the ironic admonition to "Keep out of reach of children," there is no hint on the package that these were once made exclusively for babies. On this "vanity pack," Q-tips are described as "the ultimate beauty tool."
Thus, products that result from usage drift over time can ultimately assume an identity that gives little hint of their true origins and once-primary use. The mass-produced wooden toothpick that Charles Forster introduced to Boston in the 1860s has given rise to countless fads, uses, and spinoff products, all of which ultimately owe their existence to his marketing genius, whether we realize it or not. 9
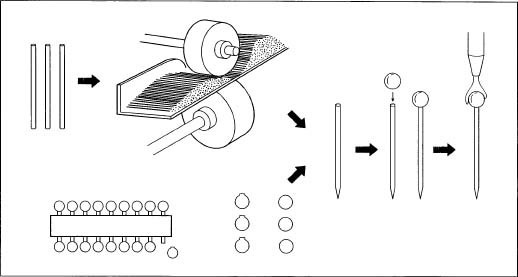
1. One-hundred-foot rolls of steel wire are unwound by means of a roll straightener. The end of each roll is threaded into the straightener, which pulls the wire flat. Rotating blades cut the wire into pre-set lengths, usually between 1-1.25 in (2.5-3.2 cm) long.
When used in this way with sweets or hors d'oeuvres, the toothpick is once again more than an item with which to pick the teeth. But on a cocktail platter, an ordinary wooden toothpick can appear too common. Thus, there has developed a plethora of "fancy" toothpicks. Gold and silver picks, with equally elegant holders, can stand up to any plate of delicacies. In Portugal, where making toothpicks by hand out of orangewood continues to be a cottage industry, larger and fancier ones called palitos especiales,complete with carved involutes and curly shafts, were considered more appropriate for special occasions. The wooden toothpick topped with colored cellophane—so often seen holding a club sandwich together—is a poor cousin to the Portuguese special.
Cocktails provided another opportunity for secondary toothpick use and further marketing. Indeed, the olive speared by a toothpick has acquired an iconic association with the martini. The toothpick enables the olive to be retrieved without getting the fingers wet, but some fastidious drinkers do not know what to do with the bare toothpick. One fellow, who did not want to put the wet pick down on the furniture, put it back into his drink—and then absentmindedly took it in with a sip. When he tried to cough it up, the thing got stuck in his nose, from where it was finally removed by an emergency-room doctor. A swallowed toothpick can be deadly if it punctures the intestines, certainly an undesirable usage drift. It was an ingested toothpick that killed the writer Sherwood Anderson, and some believe that peritonitis was also the cause of the untimely death of President Warren G. Harding, an inveterate toothpick user.
There are many other examples of usage drift, including the sticking of a toothpick into brownies baking in the oven to test if they are done. This popular secondary use is part of so many baking recipes that in supermarkets now, toothpicks often can be found shelved not among the toothbrushes and dental floss but next to the cake and brownie mixes.
Though readily promoted by manufacturers, usage drift is more often created by consumers of a product. People are natural inventors, and they are constantly finding new uses for common objects of all kinds. The best ideas propagate quickly through the culture and then become embraced by manufacturers as their own. Before there were Q-tips, young mothers wrapped a bit of cotton around the point of a toothpick and used it to clean out baby's ears and nose. This practice came to be recommended by ladies' magazines and advice columnists, and led to the invention of the Q-tip itself.
At first, the hard wooden stick terminating in soft cotton swabs suggested the toothpick connection, but today's Q-tip disguises its origins with a white paper body that blends almost seamlessly into the swab ends. The latest supply of Q-tips bought for our bathroom goes even further in removing the product from its ancestry and infancy. Except perhaps for the ironic admonition to "Keep out of reach of children," there is no hint on the package that these were once made exclusively for babies. On this "vanity pack," Q-tips are described as "the ultimate beauty tool."
Thus, products that result from usage drift over time can ultimately assume an identity that gives little hint of their true origins and once-primary use. The mass-produced wooden toothpick that Charles Forster introduced to Boston in the 1860s has given rise to countless fads, uses, and spinoff products, all of which ultimately owe their existence to his marketing genius, whether we realize it or not. 9
Background
A straight pin is a small length of stiff wire with a head at one end and a point at the other end. It is used to fasten pieces of cloth or paper together.
History
Since their ancient beginnings, human beings have devised methods for securing cloth together. Prehistoric people used thorns as pins. In ancient Egypt, pins were crafted of bronze with decorative heads. The clothes of medieval Europeans were adorned with pins of many materials including bone, ivory, silver, gold, and brass.
The use of iron wire, still applied during modern times, began as early as the fifteenth century in France. The craft of tailoring was also well-established by this time. Descriptions of a tailor's equipment from Spanish books dating back to this period included the mention of pins. A "paper of pins" became a familiar cultural phrase, signifying the possessions of the simplest nature.
At the dawn of the Industrial Revolution in the eighteenth century, noted economist Adam Smith employed the imagery of a pin factory as the perfect example of the intricate division of labor. In his book, Wealth of Nations, published in 1776, Smith described how one worker drew out the wire, another straightened it, a third cut the wire, the fourth sharpened one end, and another worker ground the opposite end for the attachment of the head. At the end of the process, the pins were polished and inserted into paper packets. These early pin factories produced just under 5,000 pins per day.
Attaching the heads presented a particular challenge. In the early to mid-1800s, American inventors Seth Hunt and John Ireland Howe and British inventors Lemuel Wright and Daniel Foote-Taylor patented machines that produced pins with a solid head from a single piece of wire. American Samuel Slocum also invented a similar machine but did not patent it. In spite of not having an official claim to this invention, the pins manufactured in Slocum's Poughkeepsie, New York factory became known as Poughkeepsie pins.
A physician by profession, Howe also liked to tinker with machinery. After watching the inmate/patients at the New York Alms House laboriously make pins by hand, he began to explore ideas for a pin-making machine. Howe enlisted the help of a printer press designer named Robert Hoe. Howe obtained a patent for his machine in June of 1832. After the machine was exhibited at the American Institute Fair in New York City, Howe was awarded a silver medal for his contribution to manufacturing.
In December of 1835, Howe formed the Howe Manufacturing Company, which was soon turning out about 70,000 pins daily. However, the packaging step slowed down the process. Workers had to manually insert the pins into paper or cards. In 1843, with the help of his employees, Howe developed a machine that crimped the paper and then inserted the pins.

Steel wire is cut and sharpened at one end. Raw plastic peal heads are then stamped onto the blunt end.emery grit. Today this bag is known as the pin cushion.
Raw Materials
Blunt wire with an international steel regulation of ISR 9002 is generally used to make straight pins. To create the wire, a bar of steel is heated to a temperature of 2,200°F (1,200°C), rolled into a long thin rod, coiled, and then allowed to cool. The heating causes an oxide coating to form on the wire. To remove this coating, the wire is immersed in an acid bath, then rinsed in water.
The cleaned wire is inserted into a drawing block that pulls the wire through a die whose opening is smaller in diameter than that of the wire. Thus the wire is reduced in diameter and increased in length. The drawing is undertaken several times until the desired diameter is obtained. As it comes off of the last stage of drawing, the steel is coiled. The dies are coated with grease or soap to protect the wire as it passes through. This lubrication process also removes defects and gives the wire a smooth finish.
Nickel is a silvery chemical element extracted from the earth's crust. It is combined with sulfate to create a solution for coating the pins to keep them from rusting.
Design
The straight pin was designed to provide a simple function, secure two or more objects together. The design is relatively simple and unchanged. The sharp tip allows penetration through materials such as cloth and paper. The head of the pin stops the entire body from slipping through the hole created by the tip, thus creating a temporary bond.
The Manufacturing Process
- In the modern pin manufacturing plant, hundreds of thousands of pins are produced daily. Although several United States companies produce and sell straight pins, virtually all of the manufacturing plants are in Asia.
1. One-hundred-foot rolls of steel wire are unwound by means of a roll straightener. The end of each roll is threaded into the straightener, which pulls the wire flat. Rotating blades cut the wire into pre-set lengths, usually between 1-1.25 in (2.5-3.2 cm) long.
2. The cut wire travels via conveyer belt to the next station where the heads are "stamped" on. One end of the wire is slammed against a block. This sharp blow causes the end to mushroom out from the shank of the wire and create a flattened head.
The pins are loaded into a circular cavity where they are hung by their heads over large grinding wheels. The grinding wheels spin the pins around to sharpen them.
The pins are loaded into a circular cavity where they are hung by their heads over large grinding wheels. The grinding wheels spin the pins around to sharpen them.
3. To ensure a strong bond between the pins and the plating solution, the pins are cleaned by dipping them in an acid solution. They are then are placed in a rack and lowered into large electroplating tanks filled with a plating solution such as nickel-sulfate. The pin rack is connected to the negative terminal of an external source of electricity. A second conductor is connected to the plating solution. A steady, direct electrical current of a low voltage, between one to six volts, is passed through the tanks. This causes the plating solution to coat the pins and give them a shiny finish.
4. The shined pins are mechanically packed in pre-ordered amounts into plastic clamshell boxes or blister packs. Bar codes are mechanically affixed to each box or pack. The individual containers are then hand-packed into cartons for shipping.
Quality Control
When the wire arrives at the straight pin factory, it is inspected for physical properties such as tensile strength and brightness. Machinery is regularly checked while they are running. Samples are drained off from each electroplating bath and sent to on-site labs. A sample of pins is pulled from each batch of 20,000 and checked again for tensile strength and brightness. The count and weight of each batch is checked before and after processing.
Byproducts/Waste
The electroplating bath creates a toxic waste product that has serious implications for the environment. Companies that employ electroplating techniques are strictly regulated by the United States Environmental Protection Agency (EPA). The concentrations of metal in the electroplating bath must be removed or disposed of in a prescribed manner. The used solution or waste water cannot be emptied into a septic system or storm water sewer. Companies must use an authorized waste transporter and the containers must meet federal United States Department of Transportation packaging standards
Chromium, one of nearly 200 toxic chemicals regulated by the federal Clean Air Act, is released into the air during the electroplating process. Plants must apply for and be granted an air pollution control permit. To qualify for the permit, plants must meet standards that regulate the amount of emissions that are allowed per day, work practices, performance testing, monitoring, record keeping, and reporting.
One way in which manufacturers reduce the incidence of these pollutants is to limit the dragout and the use of water. Dragout is any solution that escapes from the electroplating solution. By allowing the pins and pin rack to drain completely over the bath, the dragout can be substantially cut. Drain boards between the process tanks and the rinse tanks catches any solution still remaining on the parts and the product.
Spray rinses installed over the baths wash the dragout directly back into the bath. Employing spray rinses rather than continuously flowing water also reduces the incidence of pollutants.
The Future
Quality Control
When the wire arrives at the straight pin factory, it is inspected for physical properties such as tensile strength and brightness. Machinery is regularly checked while they are running. Samples are drained off from each electroplating bath and sent to on-site labs. A sample of pins is pulled from each batch of 20,000 and checked again for tensile strength and brightness. The count and weight of each batch is checked before and after processing.
Byproducts/Waste
The electroplating bath creates a toxic waste product that has serious implications for the environment. Companies that employ electroplating techniques are strictly regulated by the United States Environmental Protection Agency (EPA). The concentrations of metal in the electroplating bath must be removed or disposed of in a prescribed manner. The used solution or waste water cannot be emptied into a septic system or storm water sewer. Companies must use an authorized waste transporter and the containers must meet federal United States Department of Transportation packaging standards
Chromium, one of nearly 200 toxic chemicals regulated by the federal Clean Air Act, is released into the air during the electroplating process. Plants must apply for and be granted an air pollution control permit. To qualify for the permit, plants must meet standards that regulate the amount of emissions that are allowed per day, work practices, performance testing, monitoring, record keeping, and reporting.
One way in which manufacturers reduce the incidence of these pollutants is to limit the dragout and the use of water. Dragout is any solution that escapes from the electroplating solution. By allowing the pins and pin rack to drain completely over the bath, the dragout can be substantially cut. Drain boards between the process tanks and the rinse tanks catches any solution still remaining on the parts and the product.
Spray rinses installed over the baths wash the dragout directly back into the bath. Employing spray rinses rather than continuously flowing water also reduces the incidence of pollutants.
The Future
The straight pin has gone relatively unchanged for years and few improvements have been made. The head of the pin can now be either solid metal or plastic. Its use to bind paper together has been replaced by the stapler, but despite its simplicity, the straight pin is the first choice for a temporary way to bind cloth. 10
Sources:
8 "Manufacture of Roll Tar Paper." Chestofbooks.com. N.p., n.d. Web. 28 Nov. 2012.
9 Petroski, Henry. "Stick Figure." Slate.com. The Slate Group, 31 Oct. 2007. Web. 29 Nov. 2012.
10 "Straight Pin." Madehow.com. Advameg Inc, n.d. Web. 29 Nov. 2012.
Image from
http://www.madehow.com/Volume-7/Straight-Pin.html#b
http://www.slate.com/articles/business_and_tech/design/2007/10/stick_figure.single.html
http://www.theatlantic.com/business/archive/2011/11/the-amazing-history-and-the-strange-invention-of-the-bendy-straw/248923/
http://www.madehow.com/Volume-5/Glue.html#b
http://www.elmers.com/product/detail/E1322?filterPath=craft
http://ecogardensupply.com/Mylar-and-Lighting-Accessories/
http://www.twinsupply.com/janitorial/products.asp?cat=297
http://www.constructionweekonline.com/article-7590-uae-glass-firm-to-boost-exports-by-583/#.ULwkHqAqiFI
http://felixandwinnie.blogspot.com/2011/05/tar-paper-dinosaur-dies-in-tar-pit-tara.html
http://www.flickr.com/photos/debrabooth/348304359/
http://www.picturenation.co.uk/view/info/326794/stainless-steel-pins?page=0&catid=176
http://www.aaabalingandstrapping.com/index.php?
http://www.aaabalingandstrapping.com/index.php?main_page=product_info&cPath=5_56&products_id=218
http://yaladesigns.blogspot.com/2011/08/top-10-things-green-america-says-to.html
Sources:
1 Thompson, Derek. "The Amazing History and the Strange Invention of the Bendy Straw." The Atlantic. The Atlantic Monthly Group, 22 Nov. 2011. Web. 28 Nov. 2012.
2 Holmes, Gillian. "Glue." Madehow.com. Advameg Inc, n.d. Web. 28 Nov. 2012.
3 Hussey, Ricky. "History of Mylar Sheets and Plastics." Articles Factory. Articles Factory, 2 Mar. 2009. Web. 28 Nov. 2012.
4 "What Are Styrofoam Cups?" Wisegeek.com. Conjecture Corporation, n.d. Web. 28 Nov. 2012.
5 Busch, Anton. "When Were Styrofoam Cups Invented?" Ehow.com. Demand Media, Inc, n.d. Web. 28 Nov. 2012.
6 "A Historical Look at Glass." Glasslinks.com. PPG Industries, Inc, n.d. Web. 28 Nov. 2012.
7 "The History of Elastic and Rubber Bands." Versteegde.nl. N.p., n.d. Web. 28 Nov. 2012.
8 "Manufacture of Roll Tar Paper." Chestofbooks.com. N.p., n.d. Web. 28 Nov. 2012.
9 Petroski, Henry. "Stick Figure." Slate.com. The Slate Group, 31 Oct. 2007. Web. 29 Nov. 2012.
10 "Straight Pin." Madehow.com. Advameg Inc, n.d. Web. 29 Nov. 2012.
Image from
http://www.madehow.com/Volume-7/Straight-Pin.html#b
http://www.slate.com/articles/business_and_tech/design/2007/10/stick_figure.single.html
http://www.theatlantic.com/business/archive/2011/11/the-amazing-history-and-the-strange-invention-of-the-bendy-straw/248923/
http://www.madehow.com/Volume-5/Glue.html#b
http://www.elmers.com/product/detail/E1322?filterPath=craft
http://ecogardensupply.com/Mylar-and-Lighting-Accessories/
http://www.twinsupply.com/janitorial/products.asp?cat=297
http://www.constructionweekonline.com/article-7590-uae-glass-firm-to-boost-exports-by-583/#.ULwkHqAqiFI
http://felixandwinnie.blogspot.com/2011/05/tar-paper-dinosaur-dies-in-tar-pit-tara.html
http://www.flickr.com/photos/debrabooth/348304359/
http://www.picturenation.co.uk/view/info/326794/stainless-steel-pins?page=0&catid=176
http://www.aaabalingandstrapping.com/index.php?
http://www.aaabalingandstrapping.com/index.php?main_page=product_info&cPath=5_56&products_id=218
http://yaladesigns.blogspot.com/2011/08/top-10-things-green-america-says-to.html









Nice articles and your information valuable and good articles thank for the sharing information Thin film evaporators
ReplyDelete